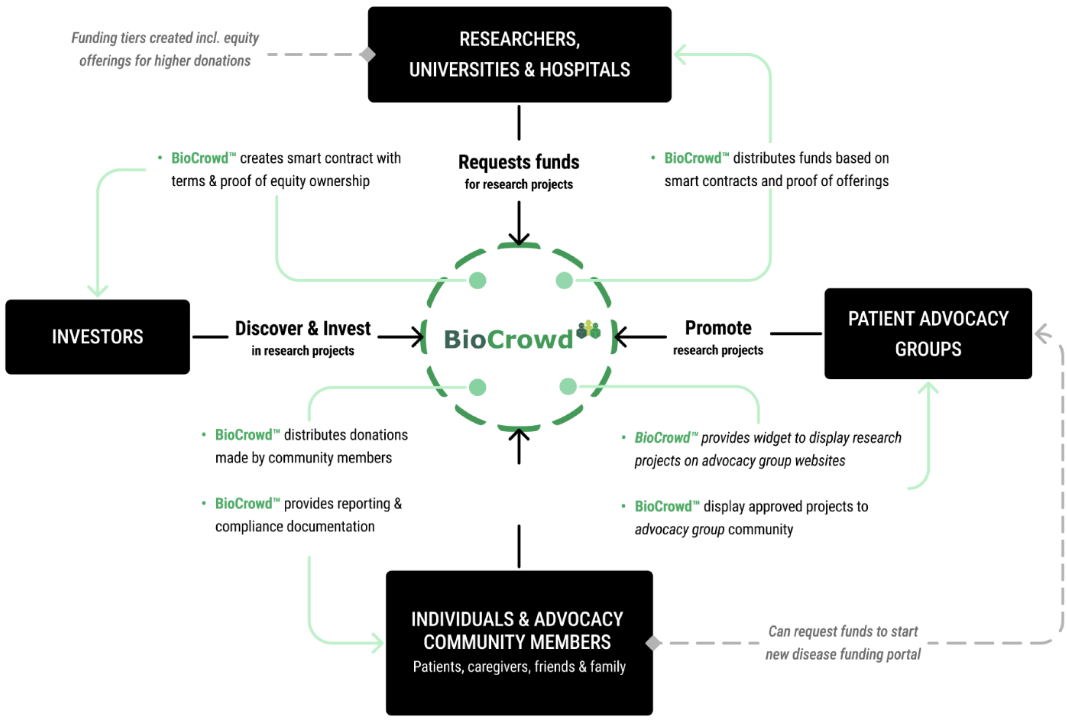
HOW IT WORKS
Milestone-Based Capital for Institution-Approved Research
BioCrowd helps hospitals, universities, and research institutes raise capital aligned to scientific and regulatory milestones.
1
Institution Approval
Projects are submitted and approved by universities, hospitals, or research institutes and defined with specific scientific, clinical, and regulatory milestones.
2
Campaign Design
BioCrowd structures:
- Milestone-based funding tranches
- Legal and compliance frameworks
- Reporting and governance models
- Donor and investor participation structures
3
Funding
Donors, foundations, and investors fund institution-approved campaigns.
Capital is released only when milestones are achieved.
4
Progress & Reporting
Institutions provide milestone-based updates on:
- Scientific progress
- Data readouts
- Regulatory steps
- Licensing or partnership discussions
Key FDA Value-Creation Milestones
BioCrowd campaigns align with major regulatory steps:
- Discovery & preclinical research
- IND application
- Phase 1 clinical trials
- Phase 2 clinical trials
- Phase 3 clinical trials
- NDA/BLA submission
- FDA approval
- Post-market monitoring
Each step:
- Reduces scientific and clinical risk
- Increases licensing or acquisition value
- Unlocks new funding or partnership opportunities
BioCrowd Introduction — Helping to Find Cures Faster at a Lower Cost